
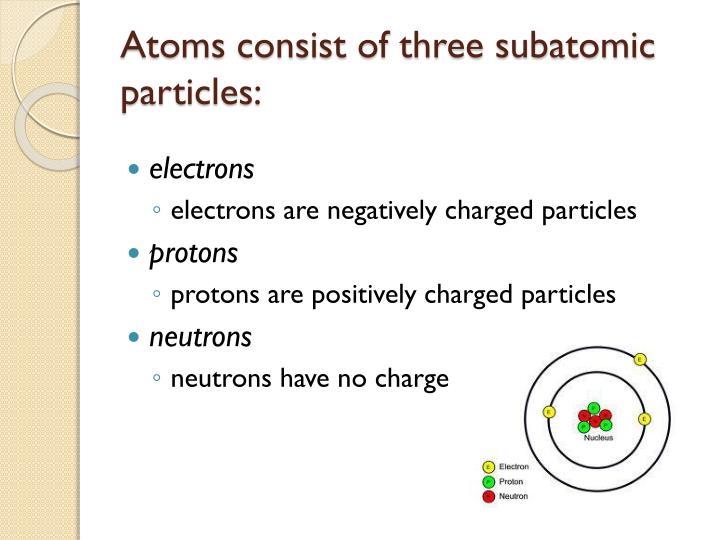
Most atoms contain all three of these types of subatomic particlesprotons, electrons, and. The attraction between the positively charged protons and negatively charged electrons holds the atom together. IN the field of quantum chromodynamics, we say that particles have a color. GENEVA, July 5 (Reuters) - Scientists working with the Large Hadron Collider (LHC) have discovered three subatomic particles never seen before as they work to unlock the building. The second, much larger, region of the atom is a cloud of electrons, negatively charged particles that orbit around the nucleus. It's just the name that we've given a quark that has certain properties. There's nothing unusually strange about the strange quark. The atoms are made up of three basic sub-atomic particles which are Proton. There's a charmed quark and a strange quark. The particles present inside the atom are called as subatomic particles. THere's nothing "up-ish" about an up quark. A typical atom consists of three subatomic particles: protons, neutrons, and electrons (as seen in the helium atom below). "Up" and "down" don't have any meaning other than to identify the type of quark. A quark can be an up quark or a down quark. We have other properties like this for other particles. It's just a characteristic that particles have or don't have, and we know what the effect is of having or not having that property. Electrons have a negative charge or a 1 charge to them. Protons have a positive charge or +1 charge on them. The three major subatomic particles present in an atom are protons, neutrons. Each of our three subatomic particles in the atom has a type of charge. "What is spin" doesn't have any deeper answer than does the question "what is charge". Diagram c whats the most variety of electrons that may occupy the third.

which predicts the existence of at least three families of quarks in nature. But there is no point in trying to answer "what is electron spin" by referring to some familiar object like a ball or a planet, because electrons are not balls or planets, they are their own thing, and you just have to accept that they have a property whose effects we understand very well, and we happen to have named it spin. to our understanding of the origin of mass of subatomic particles. Still, they have a property that works sort of like an orbit (orbitals) and they have a property that works sort of like rotation about an axis (spin). The three new types of subatomic particles, described today during a CERN seminar, aren't quite Higgs-level revelations.But they do suggest that the LHC is hot on the trail to discover still more previously unseen building blocks of the Universe. Using this concept actually helped a bit, just like Bohr's imagination of little orbits helped a bit, but again, the electrons are not like little planets: they don't revolve around the nucleus, and they don't rotate on their axes. Just as Bohr imagined that the atoms were little planets revolving around the nucleus as though it were a sun, other scientists tried to extend that idea by imagining that the little planets, just like real planets, had spin. It got named spin back when people were working with the Bohr model and trying to extend it to atoms beyond hydrogen. Spin is just a property that electrons (and other particles) have. For another layer, you can take the fact that you can never cool anything down to exactly 0K (-273.15C) (although you can get close) and so nothing will ever have 0 velocity. (1 value in a range of reals is like trying to throw a dart at a dartboard with an infinitely thin wire and hitting the wire). With this uncertainty, the velocity is almost definitely not 0. As we can't physically measure to perfect accuracy, there is an uncertainty in both measurements of the degree that we know it's probably stationary and it's probably 'over there'. So, if you know with 0 uncertainty what the velocity is, then you have no idea where it is, and all future involvement of the particle is pretty much irrelevant (how is the electron going to diffract around an atom if the electron is in a different galaxy?). The square (probability function) shows that it has an equal chance of being anywhere. If you take the infinite wavelength interpretation, then it would be nearly 0 (1/inf) but constant everywhere. Protons, neutrons, and electrons are the three main subatomic particles found in an atom. The three main subatomic particles of an atom are protons, neutrons, and electrons. such that \( P A=10 \mathrm \).Isn't to do with the fact that the velocity is not quite 0? if you know it is exactly 0 then the uncertainty in the position is infinite as well (momentum is a function of velocity, so delta P = 0 -> delta V = 0 -> delta X = inf) therefore it has an equal probability of being anywhere. If \( P A \) and \( P B \) are tangents from an outside point \( P \).Which subatomic particle is not present in an ordinary hydrogen atom?.Name the three sub-atomic particles of an atom.


 0 kommentar(er)
0 kommentar(er)
